Abstract
Introduction: CD25 is expressed on the cell surface of many lymphomas, including Hodgkin (HL), peripheral T-cell (PTCL), cutaneous T-cell (CTCL), and diffuse large B-cell lymphoma (DLBCL). ADCT-301 (camidanlumab tesirine [Cami-T]) is an antibody drug conjugate comprising a human monoclonal antibody against CD25 conjugated to a potent pyrrolobenzodiazepine dimer toxin. Here, we report interim data from the first-in-human clinical trial of Cami-T in patients (pts) with relapsed/refractory (R/R) HL and non-Hodgkin lymphoma (NHL).
Methods: This is a Phase 1, open-label, dose-escalation (Part 1) and dose-expansion (Part 2) multicenter study currently enrolling pts (≥18 years) with histologically confirmed R/R HL or NHL. The primary objectives of Part 1 are to assess the safety and tolerability, and define a maximum tolerated dose (MTD) and the recommended dose of Cami-T for Part 2. The primary objective of Part 2 will be to evaluate the safety and tolerability of Cami-T at the Part 1-recommended dose. Both parts will also assess efficacy (overall response rate [ORR; per 2014 Lugano Classification], duration of response, progression-free survival and overall survival, pharmacokinetics (PK), pharmacodynamics, and anti-drug antibody activity). Pts receive 2-hour intravenous infusions of Cami-T every 3 weeks (1 cycle). The initial cohort received a starting dose of 3 µg/kg, with subsequent cohorts enrolled at escalating doses according to a continual reassessment method, which allows expansion at different doses for different lymphoma subtypes. No intra-patient dose escalation is allowed.
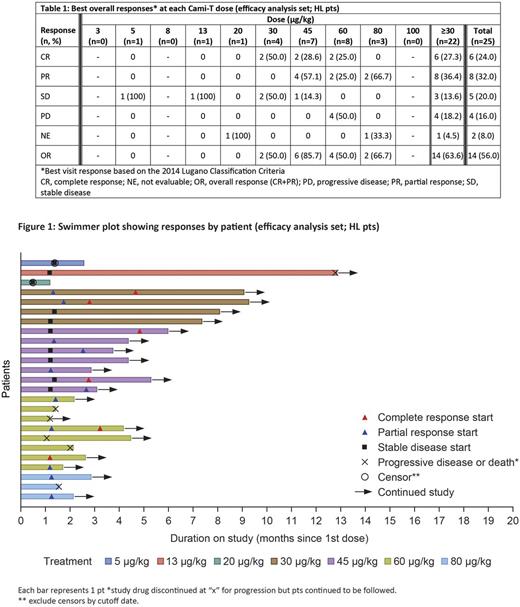
Results: As of June 28, 2017, 56 pts have been enrolled. Baseline characteristics include gender: 36 male; median age: 53.5 years (range 19-88); median number of prior therapies: 4 (range 1-14). Histological subtypes treated include HL, n=32 and NHL, n=24 (CTCL, n=6; DLBCL, n=8; mantle cell lymphoma, n=3; PTCL, n=2; follicular lymphoma, n=1; Burkitt lymphoma, n=1; other, n=3). Doses have ranged from 3 to 150 µg/kg (median number of cycles: 2 [range 1-15], with a median treatment duration of 43 days [range 2-375]). Dose-limiting toxicities have been reported in 4 pts (oral mucositis and small bowel enteritis at 20 µg/kg; elevated creatine phosphokinase at 30 µg/kg; maculopapular rash and pruritus at 30 µg/kg; lip ulceration and skin infection at 45 µg/kg). Treatment-emergent adverse events (TEAEs) were reported in 51 (91.1%) pts; most common TEAEs were fatigue (15 [26.8%] pts), maculopapular rash (14 [25.0%] pts), nausea (9 [16.1%] pts) and rash (9 [16.1%] pts). Grade ≥3 TEAEs and TEAEs leading to treatment discontinuation occurred in 24 (42.9%) and 7 (12.5%) pts, respectively. PK exposure increased with dose; accumulation was not apparent with multiple doses and Cami-T concentration in serum was below quantifiable limits before the end of the 3-week cycle. The MTD has not been reached; however, the part 2 doses for HL have been identified. Response data for pts with HL are shown in Table 1 and Figure 1. No responses were seen below 30 µg/kg. 1 pt with HL receiving 13 µg/kg remained progression-free for 45 weeks (15 cycles). 22 pts with HL have been treated with doses ≥30 µg/kg, with an ORR of 63.6% (14/22) and a complete response rate of 27.3% (6/22); ORRs by prior treatment were: 13/21 (61.9%) pts who previously received brentuximab vedotin (BV), 10/16 (62.5%) pts who previously received a checkpoint inhibitor and BV and 5/11 (45.5%) pts who had prior stem cell transplant. Responses were also seen in patients with NHL (partial response: 15.8% [3/19]; complete response: 0%). Based on these results, no further dose escalation is planned for pts with HL; it will continue for pts with NHL. The expansion cohort of pts with HL will be treated at 45, 60 and 80 µg/kg to better define the efficacy and safety of Cami-T.
Conclusions: In pts with R/R HL, active doses of Cami-T with acceptable safety profiles have been preliminarily identified during dose escalation and expansion of this study. The ORR in this heavily pretreated population is very promising and HL expansion cohorts are underway to better define the efficacy and safety of Cami-T. Dose escalation will continue to identify the MTD in NHL, with planned subtype-specific expansion cohorts at the MTD.
Study sponsored by ADC Therapeutics. http://clinicaltrials.gov/show/NCT02432235
Horwitz: Forty-Seven: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Aileron Therapeutics: Research Funding; BMS: Consultancy; Millenium/Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Mundipharma: Consultancy; ADCT Therapeutics: Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; HUYA: Consultancy; Seattle Genetics: Consultancy, Research Funding. Hamadani: Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding; Celgene, Cellerant, Jansen, MedImmune: Consultancy; Sanofi Genzyme: Research Funding, Speakers Bureau. Fanale: AMGEN: Membership on an entity's Board of Directors or advisory committees; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GENENTECH: Research Funding; TAKEDA: Honoraria, Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MOLECULAR TEMPLATES: Research Funding; ADC THERAPEUTICS: Research Funding; TAKEDA: Honoraria, Research Funding; ONYX: Research Funding; ADC THERAPEUTICS: Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GENENTECH: Research Funding; MOLECULAR TEMPLATES: Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; ONYX: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding. Feingold: ADC Therapeutics: Employment, Other: Potential equity interest. Spira: ADC Therapeutics: Research Funding. Fields: Takeda, Roche, MSD: Consultancy, Honoraria; ADC Therapeutics: Research Funding. Menne: Amgen, Takeda, Gilead: Consultancy, Honoraria; ADC Therapeutics: Research Funding. Karnad: ADC Therapeutics: Research Funding. Moskowitz: Takeda: Honoraria; Bristol Myers-Squibb: Consultancy, Research Funding; Seattle Genetics: Honoraria, Research Funding; ADC Therapeutics: Research Funding; Incyte: Research Funding. Hildyard: ADC Therapeutics: Research Funding. He: ADC Therapeutics: Employment, Other: Potential equity interest. Boni: ADC Therapeutics: Employment, Other: Potential equity interest. Collins: ADC Therapeutics: Research Funding; Celleron: Consultancy; Takeda: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.